Illustrative example - Perinatal care trial
Figure 2 presents a summary of all the staff that will be involved in data collection. All hospitals will receive one computer that will be used only for data management and operated by the data manager. Intervention hospitals will receive a second computer, but this equipment will be used by birth attendants and will have no role in data collection for primary and secondary outcomes. It will be used, however, to gather data on certain process measure, only in intervention hospitals.
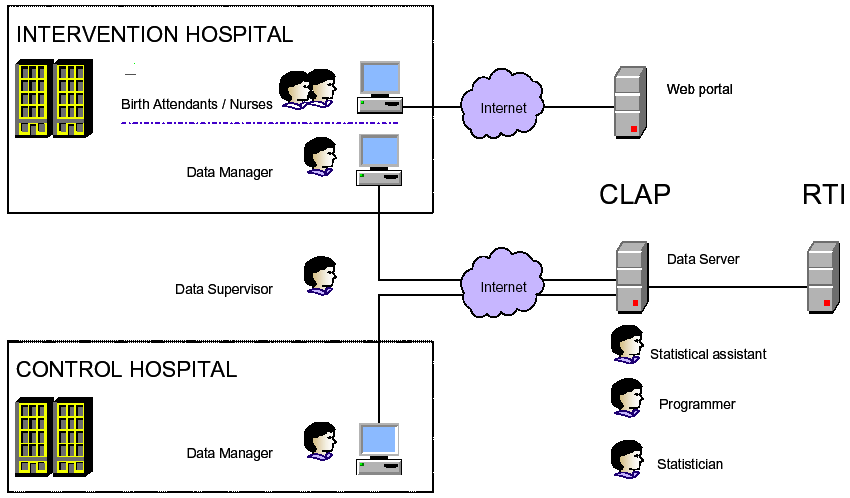
Figure 2 Staff involved in data collection.
The data collection system will be centrally coordinated at
CLAP by a statistician. The team at CLAP will include one programmer,
one statistical assistant, and two data clerks. The computer programmer
at CLAP will develop the software for data collection and validation.
The statistical assistant will carry out day-to-day data management
activities (communication with
data supervisor and data clerks at the hospitals, production of
monitoring
and validation reports, etc). For paper forms sent to CLAP, the two
data
clerks will perform two independent data entries. Two data supervisors
in
Argentina and one data supervisor in Uruguay will implement and
supervise
the data collection at the country level during the whole study period.
Data
supervisors will visit hospitals usually on a weekly basis, although
the
frequency of visits may vary according to hospital performance and
needs.
One data manager will be hired in each hospital. In most cases, this
personnel
will be one hospital employee that will work part-time for the project.
Clinical data will be collected in four periods, and each will be 1
year apart:
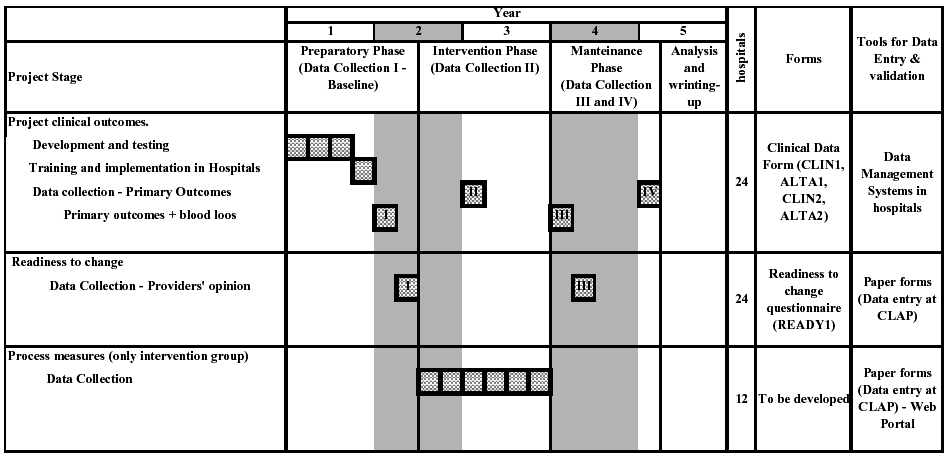
Period I. Baseline data collection: before randomization in the
preparatory phase, for primary and secondary outcomes.
Period II. Mid-intervention data collection: immediately before the
implementation of the guidelines, for primary outcomes only.
Period III. Main post-intervention data collection: immediately
following the maintenance component of the intervention, for primary
and secondary
outcomes.
Period IV. Second post-intervention data collection: 1 year after the
main post-intervention data collection, for primary outcomes only.
Questionnaire Administration
Clinical data
Data will be carbon copy from the clinical record
Questionnaires to birth attendants
Questionnaires will be administered to all birth attendants in the
participating hospitals prior to randomization (immediately after
Period I) and after
the end of the intervention (immediately after Period III).
Collection of Biological Samples
Measured total blood loss (ml)
Nurses, midwifes and physicians who are a part of the teams attending
deliveries at participating hospitals will be trained in post-partum
blood loss measurement.
Nurses and midwifes will be the main persons responsible for the
measurements.
Both the country coordinators and the data collection supervisors will
be in charge of training. A pilot study will be implemented at
the pilot hospital in Montevideo (Pereira Rossell) to assess the
acceptability of
the measuring technique.
Training study personnel in data collection
The training will be done during the preparatory phase, among other
activities. This phase will take approximately 18 months. The
Data Manager will coordinate the training in data collection
procedures. The pilot of the manual of operations for data
collection and data collection forms will be done in one hospital in
Montevideo, Uruguay, and one hospital in Buenos Aires, Argentina.
Those hospitals will not be randomized, but will
be similar to those that will be assigned to the intervention and
control
group.
Training of Biological Sample Collectors
Nurses or midwives in the labor ward of each hospital will be trained
in how to measure total blood loss in vaginal deliveries. They will be
trained to perform this measurement as a routine activity in all
vaginal deliveries during the data collection periods. Data supervisors
and Country coordinators will be in charge of the training activities
and will provide hospitals
with the standard measuring drapes.
(CLAP Trial - go to protocol)
 Checklist for data
collection
Checklist for data
collection  Questionnaire
design: asking questions with a purpose
Questionnaire
design: asking questions with a purpose