Illustrative example - Perinatal care trial
Table 4 presents the components and the schedule for data collection in the project. There are three different sets of data to be collected, and the data collection system will be different for each one. These are
-
clinical outcomes,
-
readiness to change questionnaire to health providers, and
-
process measures.
Clinical outcomes and readiness to change questionnaire includes the primary and secondary outcomes of the project and will be measured in all participating hospitals (intervention and control arm). Process measures will be used to explore aspects of the intervention implementation, and they will be measured only in the intervention hospitals.
Clinical data will be collected in four periods, and each will be 1 year apart (Table 4):
-
Period I. Baseline data collection: before randomization in the preparatory phase, for primary and secondary outcomes.
-
Period II. Mid-intervention data collection: immediately before the implementation of the guidelines, for primary outcomes only.
-
Period III. Main post-intervention data collection: immediately following the maintenance component of the intervention, for primary and secondary outcomes.
-
Period IV. Second post-intervention data collection: 1 year after the main post-intervention data collection, for primary outcomes only.
Data from period I (baseline) and III will be used for the primary analyses, and include both primary and secondary outcomes. Secondary outcomes include nonroutine procedures to measure blood loss (see below). Data collection II will be used to study trends and will be limited to primary outcomes, which can be measured without interference with the clinical processes. Data collection IV will be used to measure the sustainability of the results after the end of the intervention and will also be limited to primary outcomes to avoid interference with the clinical processes.
Table 4. Schedule for data collection.
(CLAP Trial - go to protocol)
go to protocol )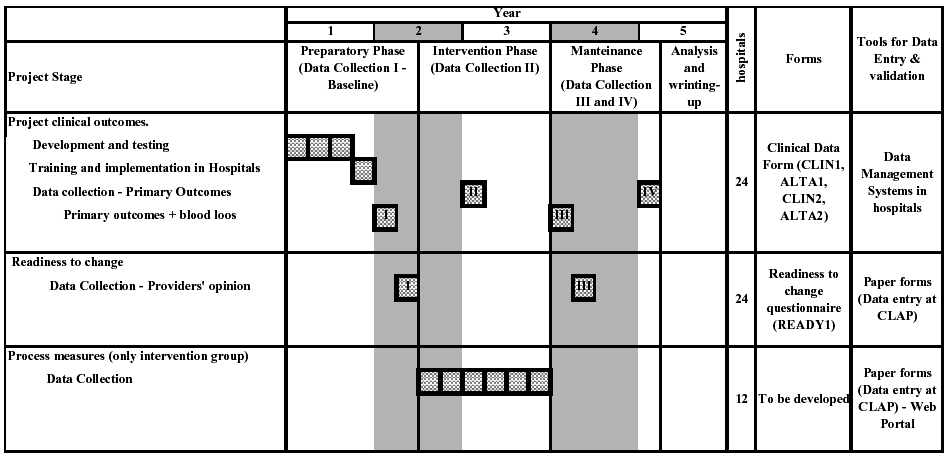
 Guidelines and checklist for
inclusion of quality of life assessment in
clinical trial protocols.
Guidelines and checklist for
inclusion of quality of life assessment in
clinical trial protocols.  UK Clearing house information sheet on ‘Clarifying desired outcomes’
UK Clearing house information sheet on ‘Clarifying desired outcomes’