 Checklist for data
backup and storage
Checklist for data
backup and storage
Whenever possible, trials should use a computerised system for data
management.
Such computerised systems are useful to keep track of the flow of
participants in the trials (e.g. recruitment, withdrawals, reason for
withdrawals, data collection, and for recording the flow of information
to/from participants (e.g. questionnaire despatch/return)). These
systems also enable quick and efficient data validation and quality
control and may also be used to set up standardised management reports
for the Trial Management Groups (see Trial management).
An effective data management system needs to be flexible and adaptable,
so that it can be tailored towards the needs of the people
collaborating in
the trial. For larger trials this may be a customised programme,
but
smaller trials may adapt off-the-shelf software. The most
suitable data management system will depend on budget constraints, the
complexity
of the trial and the technical environment. Asking the advice of
an
experienced trial programmer can be invaluable in helping to choose the
right
system. Before choosing a system considering the following
questions
may be useful:
Illustrative example - Perinatal care trial
|
|
|---|---|
|
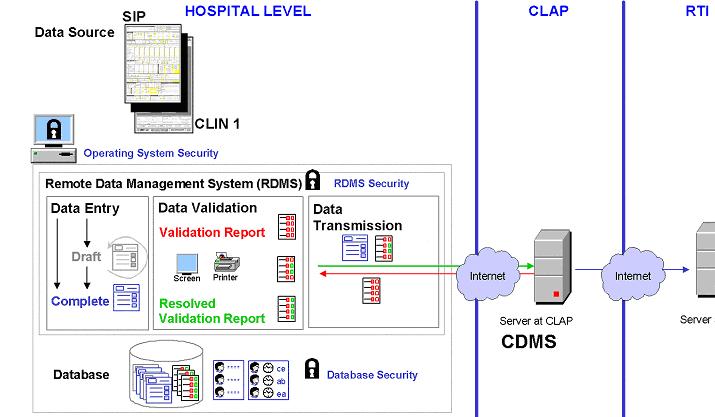
Appendix 1 contains the specifications for the development of
the software package that will be used to implement data collection
procedures at the hospitals level and at CLAP data center (see Example Data
Management Systems Specifications ). The system will have six
modules: Data Entry, Data Validation, Data Transmission, Security, and
User Management (see Example Data Management Systems
Components ). |
|
 Checklist for data
backup and storage
Checklist for data
backup and storage This checklist has been contributed by Barbara Farrell who prepared it for the second version of the Trial Management Guide.
 Example data
management system components
Example data
management system componentsThis figure was contributed by Eduardo Bergel who prepared it for
the
CLAP Trial.
 Example data
management system specifications
Example data
management system specifications This appendix was contributed by Eduardo Bergel who prepared it for the CLAP Trial.
 Epi Info
Epi Info Epi Info is a public domain software package designed for the global community of public health practitioners and researchers. It provides for easy form and database construction, data entry, and analysis with epidemiologic statistics, maps, and graphs. The primary applications within Epi Info are: